Batch RelEASY
From left to right: Peter Jan van der Veek, Kevin Bettencourt, Fabian Schuster, Ajmal Jalili, Marlou Verdurmen, Erik den Hollander.
Batch Release made easy!
I am delighted to share that the Batch RelEASY application was successfully launched in October last year. This new app enhances operational efficiency, ensures adherence to Quality Standards, and Batch RelEASY is one of the first applications to generate GxP reports live on the Self-Service Statistical Reporting Platform.
What is Batch RelEASY?
Batch RelEASY is a digital report builder tool that automates and simplifies the generation of release packages for Remicade, Stelara and Simponi (P)FB material at our Leiden and Cork Bio sites. It retrieves data from our source systems, such as SAP, MES, eLIMS, Trackwise (and soon, Comet), organizing it into reports necessary for batch release. However, it is not a transactional system for batch release. It records no decisions, activities or data. The generated reports require a ‘manual review step’, by a QA Officer, for completeness before a QP evaluates them for batch release.
The Journey – Success isn’t always immediate, and our journey is no exception. We initially attempted to use Tableau to generate release reports, which was not feasible. This led us to explore other options, eventually choosing Posit.co as our platform of choice. Posit advertises to be a better way to deploy R & python-based solutions and allows builders to use the open-source tools they know and love with the centralized management, security, and support they need.
“Effort reduces from hours to just a minute”
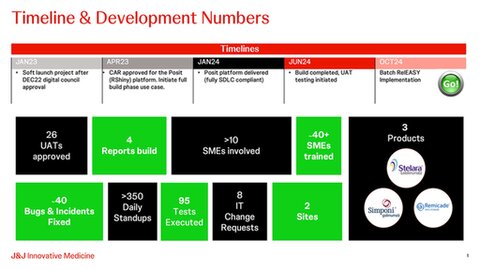
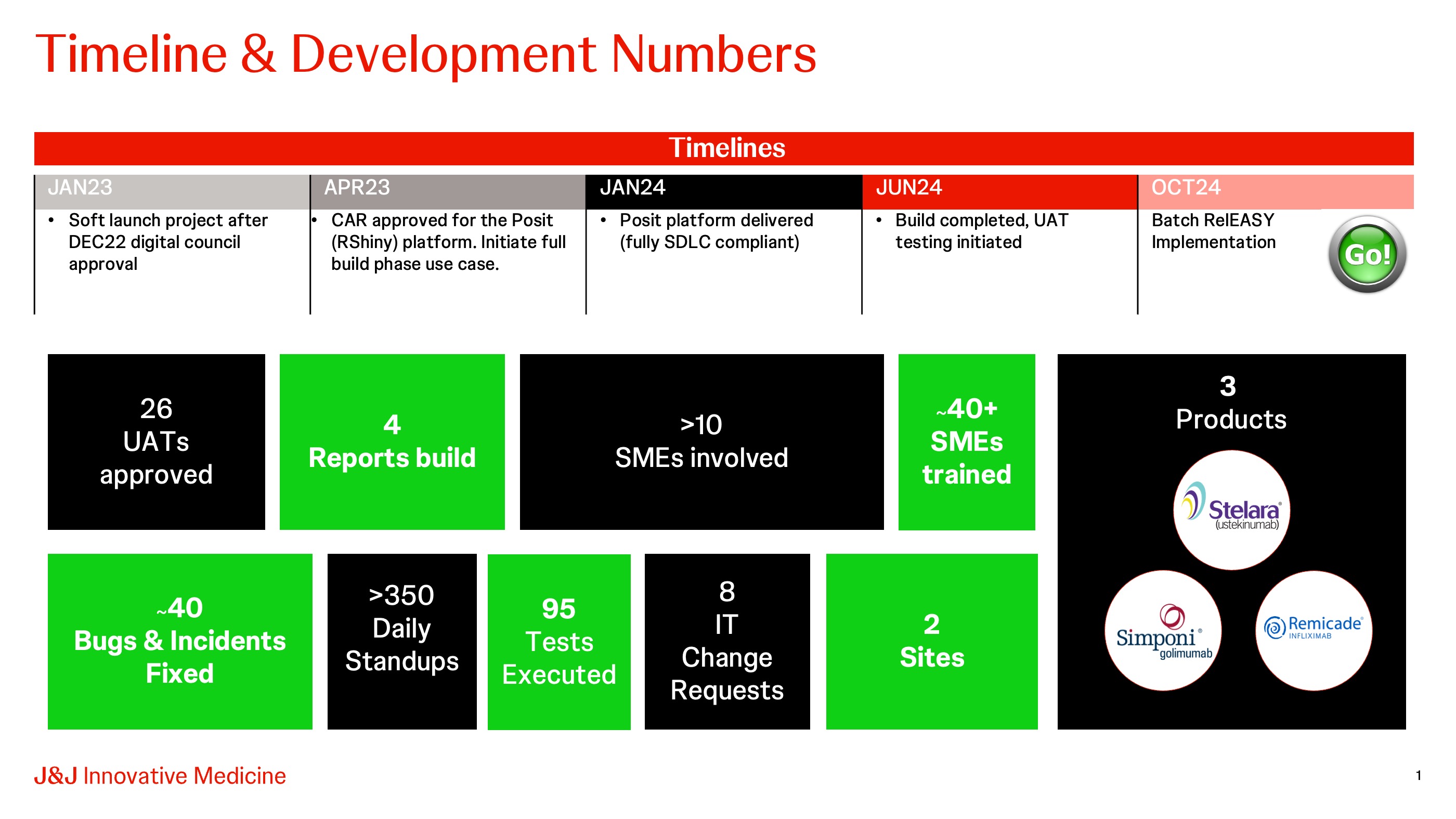
In December 2022, we received the greenlight from the global Digital & Technology council to onboard & implement Posit for Batch RelEASY development. Budget approval followed in April 2023, and from there we started building the app. The platform was rebranded within J&J as the Self-Service Statistical Reporting Platform (SSSR) and in January 2024 was delivered as a fully GxP compliant application. Over the summer period we did the user acceptance testing for Batch RelEASY and finalized the qualification effort.
Being a first mover on a new platform we faced numerous challenges but by employing agile methodology and the software development life cycle (SDLC) framework, Batch RelEASY was successfully launched at the Leiden and Cork Bio sites on October 29th.

Cork team photo
Value & Impact
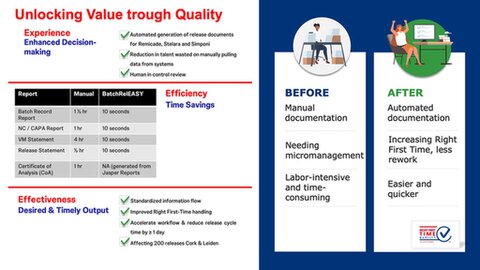
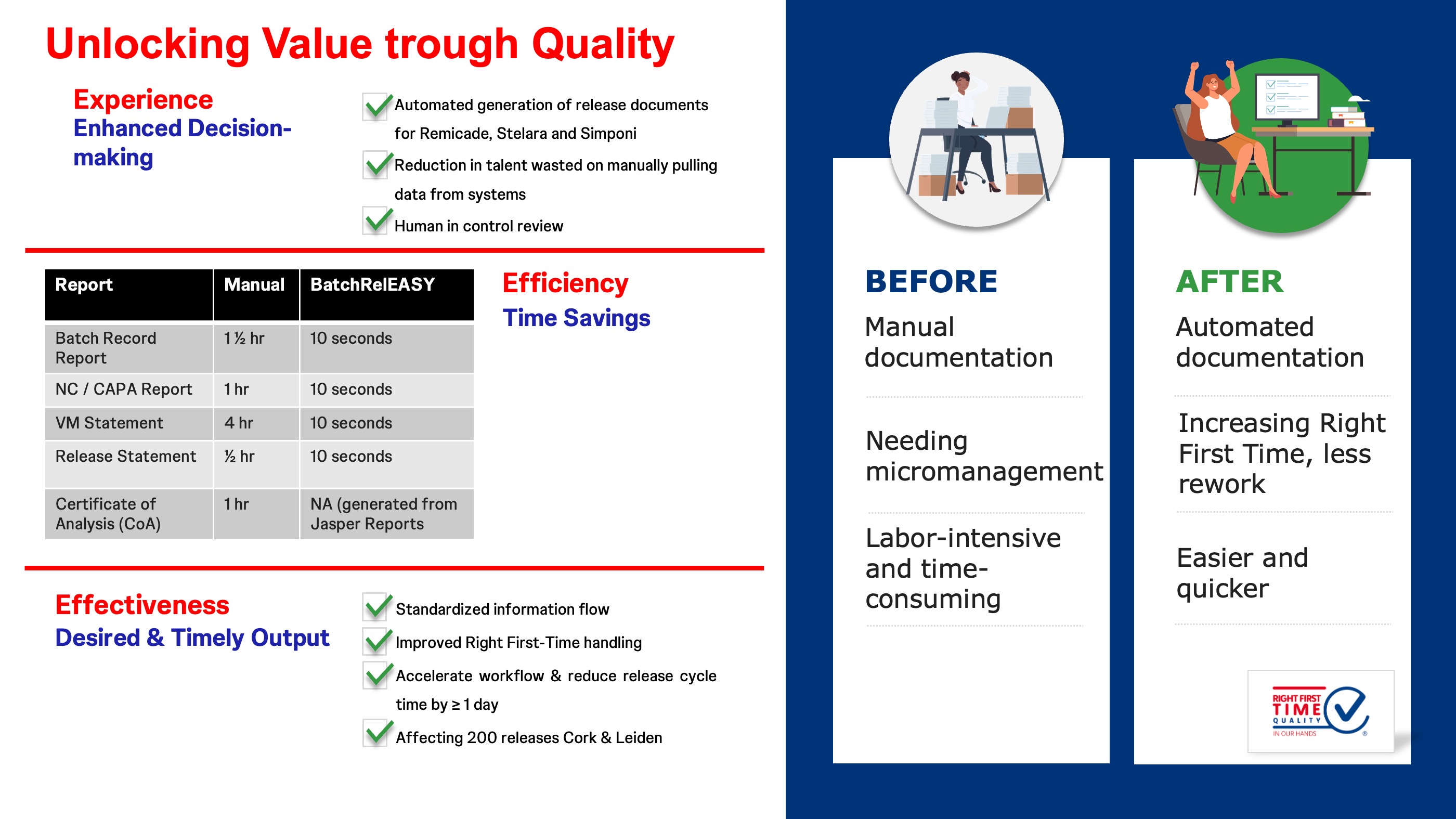
Batch RelEASY significantly enhances the Large Molecule Drug Substance Batch Release process by transforming a manual, repetitive, and labour-intensive paper-based system into a more streamlined, efficient, digital and automated process. Batch RelEASY reduces the effort required to generate all the key release reports from hours to a mere minute! This improvement not only leads to improved Right First-Time handling but also accelerates the workflow, enabling a reduction in release cycle time by a day and potentially yielding a one-time inventory savings of $1 million (not claimed). The application affects a great 200 releases annually across both Leiden and Cork Bio.
Acknowledgements
This achievement was made possible through the dedicated efforts of our core team, alongside JJT, D&T, DOXS, TQ, Business Quality, and all SMEs who provide invaluable input, tested the application and provided support from both Leiden & Cork Bio QA. A sincere thank you to everyone involved!

Erik den Hollander
Digital Change Lead Quality Digital & Data J&J Innovative Medicine
Spring break Summer party
Spring break Summer party